Other things going on recently:
ZnCr 2 O 4 Nanoparticles: Facile Synthesis, Characterization and Photocatalytic Properties |
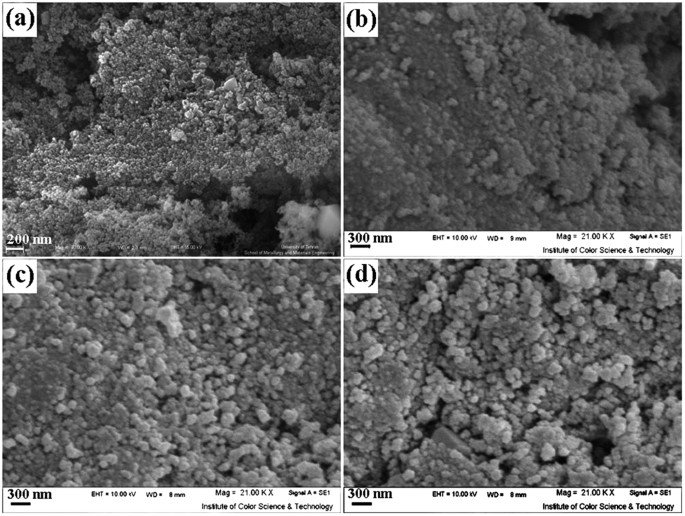
In this work, zinc chromite (ZnCr 2 O 4 ) nanostructures have been synthesized through co-precipitation method. The effect of various parameters such as alkaline agent, pH value and capping agent type was investigated on purity, particle size and morphology of samples. It was found that particle size and morphology of the products could be greatly influenced via these parameters. The synthesized products were characterized by field emission scanning electron microscopy (FESEM), X-ray diffraction (XRD), fourier transform infrared (FT-IR) spectra, X-ray energy dispersive spectroscopy (EDS), photoluminescence (PL) spectroscopy, diffuse reflectance spectroscopy (DRS) and vibrating sample magnetometry (VSM). The superhydrophilicity of the calcined oxides was investigated by wetting experiments and a sessile drop technique which carried out at room temperature in air to determine the surface and interfacial interactions. Furthermore, the photocatalytic activity of ZnCr 2 O 4 nanoparticles was confirmed by degradation of anionic dyes such as Eosin-Y and phenol red under UV light irradiation. The obtained ZnCr 2 O 4 nanoparticles exhibit a paramagnetic behavior although bulk ZnCr 2 O 4 is antiferromagnetic, this change in magnetic property can be ascribed to finite size effects.
In this paper, we describe a precipitation method to synthesis ZnCr 2 O 4 nanostructures with using Zn(NO 3 ) 2 . 6H 2 O and CrCl 3 . 6H 2 O as starting materials. The aim of this work is to synthesize ZnCr 2 O 4 nanostructures via a co-precipitation method and to investigate the effect of various parameters on their morphology. The photocatalytic activity of ZnCr 2 O 4 nanoparticles (sample 4) was evaluated by the degradation of anionic dyes such as Eosin-Y and phenol red as water pollutants. Also, the photocatalytic degradation of anionic dyes such as Eosin-Y and phenol red using spinel ZnCr 2 O 4 under UV irradiation at pH = 2–3 has been also examined.
ZnCr 2 O 4 is a spinel with cubic phase, space group of Fd/3m and space group number 227. In this work, the all of the peaks in XRD patterns of samples can be indexed to cubic phase zinc chromium oxide but JCPDSs in these patterns are not the same. The various JCPDSs in the same phases of a compound are due to grain orientations of structures, for example: the principle ZnCr 2 O 4 grain orientations are: (311), (200), (422), (440) for JCPDS = = 22–1107; (311), (220) for JCPDS = 73–1962; and (220), (311), (511), (440) for JCPDS = 01–1123. Furthermore, the crystallographic parameters in various JCPDSs aren't same, so in these patterns can be seen the different crystallographic parameters such as: a = 8.3257 in JCPDS = 22–1107; a = 8.28 in JCPDS = 73–1962; and a = 8.3200 in JCPDS = 01–1123 (cubic phase has a = b = c and α = β = γ = 90°). By considering above explanations, the various JCPDSs in same phases of a compound can be used to determine grain orientations of structures.
The band gap of ZnCr 2 O 4 synthesized in this work was estimated about 3.35 eV and 3.96 eV by UV-vis and PL spectroscopy, respectively. As shown the amount of calculated band gap of the product using these methods (Tauc equation in UV-vis and 1240/λ max in PL spectroscopy) is not same, this difference can be explained follow:
Usually, the traditional method of estimating of the band gap of semiconductor materials is based on the results of absorption spectra measurements. An evaluation of bandwidth is based only on the absorption measurements as a result of interband transitions. Fluorescence upconversion signal is the sum of two fluorescence beams, one from the sample itself (which is the actual fluorescence of molecule) and one from the excitation source. Up-converted signal is not due to the relaxation of molecular excited state or you cannot say that we have got a higher optical band gap. In case of two photon absorption, the absorption of the second photon comes from a virtual excited state and not from actual excited state (LUMO or S1). So in both cases, the band gap of the molecule is same, as obtained by UV-visible spectroscopy. So, calculation of band gap through UV-vis spectroscopy is more accurate.
Photograph of measured contact angle (θ) on rough surface of zinc chromium oxide (pellet) material (sample 4).
The two main features that dominate the magnetic properties of nanoparticles and give them various special properties are:
(a) Finite-size effects (single-domain or multi-domain structures and quantum confinement of the electrons);
In summary, ZnCr 2 O 4 nanoparticles have been successfully synthesized from Zn(NO 3 ) 2 . 6H 2 O and CrCl 3 . 6H 2 O by a simple co-precipitation method, under low temperature and ambient pressure. The fine and pure ZnCr 2 O 4 nanoparticles were produced through adjusting the pH value, appropriate alkaline and capping agents. Wettability of ZnCr 2 O 4 nanoparticles synthesized in this work, was considered by the contact angle goniometer. The contact angle (θ) was 5 ο , which indicates that oxide material was superhydrophilic in nature. In comparison to other similar works, our method is facile, simple, low cost and eco-friendly. The produced zinc chromite nanostructures can be utilized as a remarkable photocatalyst for dye degradation from waste-water such as removal of Eosin-Y and phenol red as water pollutants under UV irradiation. The degradation percentages of Eosin-Y and phenol red under UV-irradiation were calculated about 95.26 and 13.42, respectively. VSM analysis depicted that ZnCr 2 O 4 nanoparticles have a paramagnetic characteristic, although the bulk ZnCr 2 O 4 represents an antiferromagnetic behavior and this change in magnetic property can be ascribed to finite size effects in nano scale materials.
:max_bytes(150000):strip_icc()/GettyImages-22248417481-719b33eb0ca54591b2532fc82e7cabf6.jpg)
No comments:
Post a Comment